Abstract
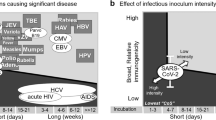
The SARS-CoV-2 epidemic is merely the most recent demonstration that our current approach to emerging zoonotic infectious disease is ineffective. SARS, MERS, Ebola, Nipah and an array of arenavirus infections sporadically spillover into human populations and are often contained only as a result of their poor transmission in human hosts, coupled with intense public health control efforts in the early stages of an emerging epidemic. It is now more apparent than ever that we need a better and more proactive approach. One possibility is to eliminate the threat of spillover before it occurs using vaccines capable of autonomously spreading through wild animal reservoirs. We are now poised to begin developing self-disseminating vaccines targeting a wide range of human pathogens, but important decisions remain about how they can be most effectively designed and used to target pathogens with a high risk of spillover and/or emergence. In this Perspective, we first review the basic epidemiological theory establishing the feasibility and utility of self-disseminating vaccines. We then outline a road map for overcoming remaining technical challenges: identifying high-risk pathogens before they emerge, optimizing vaccine design with an eye to evolution, behaviour and epidemiology, and minimizing the risk of unintended consequences.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Katy Riendeau


Katy Riendeau
Similar content being viewed by others
References
Redding, D. W., Moses, L. M., Cunningham, A. A., Wood, J. & Jones, K. E. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. Methods Ecol. Evol. 7, 646–655 (2016).
McCormick, J. B. & Fisher-Hoch, S. P. in Arenaviruses I: The Epidemiology, Molecular and Cell Biology of Arenaviruses — Current Topics in Microbiology and Immunology Vol. 262 (ed. Oldstone, M. B. A.) 75–109 (Springer, 2002).
Jonsson, C. B., Figueiredo, L. T. M. & Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23, 412–441 (2010).
Edson, D. et al. Routes of Hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS ONE 10, e0140670 (2015).
Luby, S. P., Gurley, E. S. & Jahangir Hossain, M. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 49, 1743–1748 (2009).
Georgiou, G. et al. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat. Biotechnol. 15, 29–34 (1997).
Leitner, W. W., Ying, H. & Restifo, N. P. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18, 765–777 (1999).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Rollier, C. S., Reyes-Sandoval, A., Cottingham, M. G., Ewer, K. & Hill, A. V. S. Viral vectors as vaccine platforms: deployment in sight. Curr. Opin. Immunol. 23, 377–382 (2011).
Ferretti, L. et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 368, eabb6936 (2020).
Morse, S. S. et al. Prediction and prevention of the next pandemic zoonosis. Lancet 380, 1956–1965 (2012).
Rupprecht, C. E., Hanlon, C. A. & Slate, D. in Control of Infectious Animal Diseases by Vaccination — Developments in Biologicals Vol. 119 (eds Schudel, A. & Lombard, M.) 173–184 (Karger, 2004).
Bull, J. J., Smithson, M. W. & Nuismer, S. L. Transmissible viral vaccines. Trends Microbiol. 26, 6–15 (2018).
Murphy, A. A., Redwood, A. J. & Jarvis, M. A. Self-disseminating vaccines for emerging infectious diseases. Expert Rev. Vaccines 15, 31–39 (2016).
Shellam, G. R. The potential of murine cytomegalovirus as a viral vector for immunocontraception. Reprod. Fertil. Dev. 6, 401–409 (1994).
Tyndale-Biscoe, C. H. Virus-vectored immunocontraception of feral mammals. Reprod. Fertil. Dev. 6, 281–287 (1994).
Barcena, J. et al. Horizontal transmissible protection against myxomatosis and rabbit hemorrhagic disease by using a recombinant myxoma virus. J. Virol. 74, 1114–1123 (2000).
Torres, J. M. et al. First field trial of a transmissible recombinant vaccine against myxomatosis and rabbit hemorrhagic disease. Vaccine 19, 4536–4543 (2001).
Angulo, E. & Barcena, J. Towards a unique and transmissible vaccine against myxomatosis and rabbit haemorrhagic disease for rabbit populations. Wildl. Res. 34, 567–577 (2007).
Nuismer, S. L. et al. Eradicating infectious disease using weakly transmissible vaccines. Proc. R. Soc. B 283, 20161903 (2016).
Basinski, A. J., Nuismer, S. L. & Remien, C. H. A little goes a long way: weak vaccine transmission facilitates oral vaccination campaigns against zoonotic pathogens. PLoS Negl. Trop. Dis. 13, e0007251 (2019).
Basinski, A. J. et al. Evaluating the promise of recombinant transmissible vaccines. Vaccine 36, 675–682 (2018).
Smithson, M. W., Basinki, A. J., Nuismer, S. L. & Bull, J. J. Transmissible vaccines whose dissemination rates vary through time, with applications to wildlife. Vaccine 37, 1153–1159 (2019).
Lecompte, E. et al. Mastomys natalensis and Lassa fever, West Africa. Emerg. Infect. Dis. 12, 1971–1974 (2006).
Olayemi, A. et al. New hosts of the Lassa virus. Sci. Rep. 6, 25280 (2016).
Douglass, R. J. et al. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am. J. Trop. Med. Hyg. 65, 33–41 (2001).
Luis, A. D., Douglass, R. J., Mills, J. N. & Bjornstad, O. N. The effect of seasonality, density and climate on the population dynamics of Montana deer mice, important reservoir hosts for Sin Nombre hantavirus. J. Anim. Ecol. 79, 462–470 (2010).
Viana, M. et al. Assembling evidence for identifying reservoirs of infection. Trends Ecol. Evol. 29, 270–279 (2014).
Fenton, A., Streicker, D. G., Petchey, O. L. & Pedersen, A. B. Are all hosts created equal? Partitioning host species contributions to parasite persistence in multihost communities. Am. Nat. 186, 610–622 (2015).
Fichet-Calvet, E. et al. Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector-Borne Zoonotic Dis. 7, 119–128 (2007).
Marien, J. et al. Evaluation of rodent control to fight Lassa fever based on field data and mathematical modelling. Emerg. Microbes Infect. 8, 640–649 (2019).
Towner, J. S. et al. Marburg virus infection detected in a common african bat. PLoS ONE 2, e764 (2007).
Nziza, J. et al. Coronaviruses detected in bats in close contact with humans in Rwanda. EcoHealth 17, 152–159 (2020).
Anthony, S. J. et al. Further evidence for bats as the evolutionary source of Middle East respiratory syndrome coronavirus. Mbio 8, e00373–17 (2017).
Ge, X.-Y. et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538 (2013).
Bird, B. H. & Mazet, J. A. K. Detection of emerging zoonotic pathogens: an integrated one health approach. Annu. Rev. Anim. Biosci. 6, 121–139 (2018).
Goldstein, T. et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 3, 1084–1089 (2018).
Pernet, O. et al. Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 5, 5342 (2014).
Grard, G. et al. A novel rhabdovirus associated with acute hemorrhagic fever in Central Africa. PLoS Pathog. 8, e1002924 (2012).
Han, B. A. & Drake, J. M. Future directions in analytics for infectious disease intelligence. EMBO Rep. 17, 785–789 (2016).
Han, B. A., Schmidt, J. P., Bowden, S. E. & Drake, J. M. Rodent reservoirs of future zoonotic diseases. Proc. Natl Acad. Sci. USA 112, 7039–7044 (2015).
Han, B. A. et al. Undiscovered bat hosts of filoviruses. PLoS Negl. Trop. Dis. 10, e0004815 (2016).
Guth, S., Visher, E., Boots, M. & Brook, C. E. Host phylogenetic distance drives trends in virus virulence and transmissibility across the animal-human interface. Philos. Trans. R. Soc. B 374, 20190296 (2019).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Pepin, K. M., Lass, S., Pulliam, J. R. C., Read, A. F. & Lloyd-Smith, J. O. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nat. Rev. Microbiol. 8, 802–813 (2010).
Babayan, S. A., Orton, R. J. & Streicker, D. G. Predicting reservoir hosts and arthropod vectors from evolutionary signatures in RNA virus genomes. Science 362, 577–580 (2018).
Bakker, K. M. et al. Fluorescent biomarkers demonstrate prospects for spreadable vaccines to control disease transmission in wild bats. Nat. Ecol. Evol. 3, 1697–1704 (2019).
Garnier, R., Gandon, S., Chaval, Y., Charbonnel, N. & Boulinier, T. Evidence of cross-transfer of maternal antibodies through allosuckling in a mammal: potential importance for behavioral ecology. Mamm. Biol. 78, 361–364 (2013).
Stading, B. et al. Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine. PLoS Negl. Trop. Dis. 11, e0005958 (2017).
Schreiner, C. L., Nuismer, S. L. & Basinski, A. J. When to vaccinate a fluctuating wildlife population: is timing everything? J. Appl. Ecol. 57, 307–319 (2020).
Varrelman, T. J., Basinski, A. J., Remien, C. H. & Nuismer, S. L. Transmissible vaccines in heterogeneous populations: implications for vaccine design. One Health 7, 100084 (2019).
Alizon, S., Hurford, A., Mideo, N. & Van Baalen, M. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J. Evol. Biol. 22, 245–259 (2009).
Kew, O. M., Sutter, R. W., de Gourville, E. M., Dowdle, W. R. & Pallansch, M. A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59, 587–635 (2005).
Bull, J. J. Evolutionary reversion of live viral vaccines: can genetic engineering subdue it? Virus Evol. 1, vev005 (2015).
Lauring, A. S., Jones, J. O. & Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 28, 573–579 (2010).
Nuismer, S. L., Basinski, A. & Bull, J. J. Evolution and containment of transmissible recombinant vector vaccines. Evol. Appl. 12, 1595–1609 (2019).
Kew, O. M. et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 82, 16–23 (2004).
Hampson, K. et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 9, e0003709 (2015).
Cost of the Ebola Epidemic (US Centers for Disease Control and Prevention, 2020); https://go.nature.com/38iF7cg
Forum on Microbial Threats Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary (National Academies Press, 2004).
Acknowledgements
We thank K. Riendeau for developing Figs. 1 and 3. S.L.N. and J.J.B. were supported by National Institutes of Health (NIH) grant no. R01GM122079.
Author information
Authors and Affiliations
Contributions
S.L.N. and J.J.B. conceived of this Perspective and contributed to writing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nuismer, S.L., Bull, J.J. Self-disseminating vaccines to suppress zoonoses. Nat Ecol Evol 4, 1168–1173 (2020). https://doi.org/10.1038/s41559-020-1254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1254-y